The word “sepsis” was derived from the ancient Greek for rotten flesh and putrefaction. Sir William Osler was the first to recognize that “except on few occasions, the patient appears to die from the body’s response to infection rather than from the infection.”
In 1914, Schottmueller wrote, "Septicemia is a state of microbial invasion from a portal of entry into the blood stream which causes sign of illness." The definition did not change significantly over the years because sepsis and septicemia were considered to refer to a number of ill-defined clinical conditions in addition to bacteriemia. In practice, the terms were often used interchangeably; however, less than one half of the patients who have signs and symptoms of sepsis have positive blood culture results.
In the late 1960s, several reports appeared describing remote organ failure (eg, pulmonary failure, liver failure) as a complication of severe sepsis. In 1975, a classic editorial by Baue was entitled "Multiple, progressive or sequential systems failure, a syndrome of the 1970s." This concept was formulated as the basis of a new clinical syndrome. Several terms were cloned thereafter, such as multiple organ failure, multiple system organ failure, and multiple organ system failure, to describe this evolving clinical syndrome of otherwise unexplained progressive physiological failure of several interdependent organ systems. More recently, the term multiple organ dysfunction syndrome (MODS) has been proposed as a more appropriate description.
The American College of Chest Physicians/Society of Critical Care Medicine Consensus Panel developed definitions of the various stages of sepsis, which can be summarized as follows:
Sepsis has been referred to as a process of malignant intravascular inflammation. Normally, a potent, complex, immunologic cascade ensures a prompt protective response to microorganism invasion in humans. A deficient immunologic defense may allow infection to become established; however, an excessive or poorly regulated response may harm the host through maladaptive release of indigenously generated inflammatory compounds.
Lipid A and other bacterial products release cytokines and other immune modulators that mediate the clinical manifestations of sepsis. Interleukins, tumor necrosis factor-alpha (TNF-alpha), interferon gamma, and other colony-stimulating factors are produced rapidly within minutes or hours after interactions of monocytes and macrophages with lipid A. Inflammatory mediators release becomes a self-stimulating process (an autocrine), and release of other inflammatory mediators, including interleukin-1 (IL-1), platelet activating factor, IL-2, IL-6, IL-8, IL-10, INF, and nitric oxide, further increases cytokine levels. This leads to continued activation of polymorphonuclear leukocytes (PMNs), macrophages, and lymphocytes; proinflammatory mediators recruit more of these cells (a paracrine process). All of these processes create a state of destructive immunologic dissonance.
Sepsis is described as an autodestructive process that permits extension of the normal pathophysiologic response to infection to involve otherwise normal tissues and results in MODS.
Significant derangement in autoregulation of circulation is typical of sepsis. Vasoactive mediators cause vasodilatation and increase the microvascular permeability at the site of infection. Nitric oxide plays a central role in the vasodilatation of septic shock. Also, impaired secretion of vasopressin may occur, which may permit persistence of vasodilatation.
Central circulation: Changes in both systolic and diastolic ventricular performance occur in sepsis. Through the use of the Frank Starling mechanism, cardiac output often is increased to maintain the BP in the presence of systemic vasodilatation. Patients with preexisting cardiac disease are unable to increase their cardiac output appropriately.
Regional circulation: Sepsis interferes with the normal distribution of systemic blood flow to organ systems; therefore, core organs may not receive appropriate oxygen delivery leading to what is knows as regional hypoperfusion.
Microcirculation is the key target organ for injury in sepsis syndrome. A decrease in the number of functional capillaries causes an inability to extract oxygen maximally, which is caused by intrinsic and extrinsic compression of capillaries and plugging of the capillary lumen by blood cells. Increased endothelial permeability leads to widespread tissue edema of protein-rich fluid.
Septic shock and the systemic inflammatory response is characterized by reversible myocardial depression, which can prove resistant to catecholamine and fluid administration. Circulating "myocardial depressant factor"—probably representing the synergistic effects of TNFα, IL-1β, other cytokines, and nitric oxide—is implicated in pathogenesis. Macrovascular myocardial ischemia and hypoperfusion are unlikely contributors. In severe sepsis and septic shock, microcirculatory dysfunction, and mitochondrial depression cause regional tissue distress, therefore, regional hypoxia persists. This condition is termed microcirculatory and mitochondrial distress syndrome (MMDS).[1 ]Sepsis-induced inflammatory autoregulatory dysfunction persists and oxygen need is not matched by supply, leading to multiorgan system dysfunction.
Redistribution of intravascular fluid volume resulting from reduced arterial vascular tone, diminished venous return from venous dilation, and release of myocardial depressant substances causes hypotension.
Liver failure or "shock liver" can manifest by elevation of liver enzymes and bilirubins, coagulation defects, and failure to excrete toxins such as ammonia, which lead to worsening encephalopathy.
Symptoms of sepsis are usually nonspecific and include fever, chills, and constitutional symptoms of fatigue, malaise, anxiety, or confusion.[4 ]These symptoms are not pathognomonic for infection and may be observed in a wide variety of noninfectious inflammatory conditions. They may be absent in serious infections, especially in elderly individuals.
Physical examination notes the general condition of the patient first. Observe the overall hemodynamic condition to search for signs of hyperperfusion. Look for signs suggestive of a focal infection. An acutely ill, toxic appearance is a common feature in serious infections.
Two well-defined forms of multiorgan dysfunction syndrome exist. In both, the development of acute lung injury or ARDS is of key importance to the natural history. ARDS is the earliest manifestation in all cases.
The treatment of patients with septic shock consists of the following 3 major goals: (1) Resuscitate the patient from septic shock using supportive measures to correct hypoxia, hypotension, and impaired tissue oxygenation. (2) Identify the source of infection and treat with antimicrobial therapy, surgery, or both. (3) Maintain adequate organ system function guided by cardiovascular monitoring and interrupt the pathogenesis of multiorgan system dysfunction.
The principles in the management of septic shock based on current literature include the following components:
Take patients with infected foci to surgery after initial resuscitation and administration of antibiotics for definitive surgical treatment. Little is gained by spending hours stabilizing the patient when an infected focus persists.
The proven medical treatments for septic shock are restoration of intravascular volume and broad-spectrum empirical antibiotic coverage. All other medical therapies, while theoretically attractive, have not reduced morbidity or mortality.
1-2 L IV initially, with reassessment of hemodynamic response; amounts required during the first few hours typically are 4-5 L
Pediatric
Not established
Interactions
None reported
Contraindications
Pulmonary edema in which added fluid promotes more edema and may lead to development of ARDS
Precautions
Pregnancy
A - Fetal risk not revealed in controlled studies in humans
Precautions
Closely monitor cardiovascular and pulmonary function; stop fluids when the desired hemodynamic response is observed or pulmonary edema develops; interstitial edema is a major complication; edema in an extremity is unsightly but not a significant complication; edema in brain or lungs is potentially fatal
Colloids
Resuscitation fluids used because they provide an oncotically active substance that expands plasma volume to a greater degree than isotonic crystalloids and reduces the tendency to pulmonary and cerebral edema. Approximately 50% of the administered colloid remains intravascular.
Albumin 5% (Albuminar, Albunex)
Used for treatment of certain types of shock or impending shock. Useful for plasma volume expansion and maintenance of cardiac output.
Solution of normal saline and 5% albumin is available for volume resuscitation.
Dosing
Adult
250-500 mL IV over 20-30 min, with reassessment of hemodynamic response
Pediatric
Not established
Interactions
None reported
Contraindications
Documented hypersensitivity; pulmonary edema; protein load of 5% albumin
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
No proven benefit of colloid resuscitation over isotonic crystalloids is known; protein load tends to exacerbate renal insufficiency, a potential complication of septic shock; studies document an increased incidence of renal failure in patients with colloid resuscitation
Antibiotics
Empirical antibiotics that cover the infecting organism and are started early are the only other proven medical treatment for septic shock. In order to provide the necessary coverage, broad-spectrum and/or multiple antibiotics are started. Monotherapy is possible in adults who are not immunocompromised with either antipseudomonal penicillin or a carbapenem. Combination therapy in adults involves 1 of the following: a third-generation cephalosporin plus anaerobic coverage (clindamycin or metronidazole) or a fluoroquinolone plus clindamycin. Administer all initial antibiotics intravenously in patients with septic shock.
Cefotaxime (Claforan)
Used for treatment of septicemia. Also used for treatment of gynecologic infections caused by susceptible organisms. Third-generation cephalosporin with enhanced gram-negative coverage, especially to Escherichia coli, Proteus species, and Klebsiella species. Has variable activity against Pseudomonas species.
Dosing
Adult
1-2 g IV q4h
Pediatric
Not established
Interactions
Probenecid may decrease cefotaxime clearance, causing an increase in cefotaxime levels; furosemide and aminoglycosides may increase nephrotoxicity when used concurrently
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Adjust dose in severe renal impairment; associated with severe colitis
Ceftriaxone (Rocephin)
Used because of an increasing prevalence of penicillinase-producing microorganisms. Inhibits bacterial cell wall synthesis by binding to 1 or more of the penicillin-binding proteins. Bacteria eventually lyse due to the ongoing activity of cell wall autolytic enzymes while cell wall assembly is arrested.
Dosing
Adult
1 g IV q6-12h
Pediatric
Not established
Interactions
Probenecid may decrease ceftriaxone clearance, causing an increase in ceftriaxone levels; ethacrynic acid, furosemide, and aminoglycosides may increase nephrotoxicity when used concurrently
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Adjust dose in renal impairment; use with caution in breastfeeding women and in patients allergic to penicillin
Cefuroxime (Zinacef)
Second-generation cephalosporin that maintains gram-positive activity of the first-generation cephalosporins and adds activity against E coli, Klebsiella pneumoniae, Proteus mirabilis, Haemophilus influenzae, and Moraxella catarrhalis. Condition of the patient, severity of the infection, and susceptibility of the microorganism determine proper dose and route of administration.
Dosing
Adult
1.5 g IV q8h
Pediatric
Not established
Interactions
Alcoholic beverages consumed concurrently within <72 h after taking cefuroxime may produce acute alcohol intolerance (disulfiramlike reaction); hypoprothrombinemic effects of anticoagulants may be increased by cephalosporins with the methyltetrazolethiol side chain (eg, cefuroxime); monitor renal function in patients receiving potent diuretics (eg, loop diuretics), risk of nephrotoxicity may be increased; aminoglycoside nephrotoxicity may potentiate cefuroxime effects in the kidney when used concurrently, monitor renal function closely
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Administer one half the dose to patients with creatinine clearance of 10-30 mL/min; administer one fourth the dose to patients with a creatinine clearance of <10 mL/min; use of antibiotics for prolonged periods of time or repeated therapy may result in bacterial or fungal overgrowth of nonsusceptible organisms that may lead to a secondary infection, take appropriate measures if superinfection occurs
Ticarcillin/clavulanate (Timentin)
Antipseudomonal penicillin plus a beta-lactamase inhibitor that provides coverage against most gram-positives (variable coverage against Staphylococcus epidermidis and none against methicillin-resistant Staphylococcus aureus [MRSA]), most gram-negative organisms, and most anaerobes.
Dosing
Adult
3.1 g (ticarcillin 3 g and claculanate 0.1 g) IV q4-6h
Pediatric
Not established
Interactions
Tetracyclines may decrease effects; high concentrations may physically inactivate aminoglycosides if administered in the same IV line; probenecid may increase penicillin levels; effects when administered concurrently with aminoglycosides are synergistic
Contraindications
Documented hypersensitivity; severe pneumonia; do not treat bacteremia, pericarditis, emphysema, meningitis, and purulent or septic arthritis with an oral penicillin during acute stage
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Perform CBCs prior to initiation of therapy and at least weekly during therapy; monitor for liver function abnormalities by measuring AST and ALT during therapy; perform urinalysis, BUN, and creatinine determinations during therapy, and adjust dose if these values become elevated; if renal impairment is known or suspected, adjust dose and monitor blood levels; these measures avoid possible neurotoxic reactions
Piperacillin/tazobactam (Zosyn)
Inhibits the biosynthesis of cell wall mucopeptide and is effective during the stage of active multiplication. Has antipseudomonal activity.
Dosing
Adult
3/0.375 g (piperacillin 3 g and tazobactam 0.375 g) IV q6h
Pediatric
Not established
Interactions
Tetracyclines may decrease the effects; high concentrations may physically inactivate aminoglycosides; probenecid may increase penicillin levels; effects when administered concurrently with aminoglycosides are synergistic
Contraindications
Documented hypersensitivity; do not treat severe pneumonia, bacteremia, pericarditis, emphysema, meningitis, and purulent or septic arthritis with an oral penicillin during the acute stage
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Perform CBCs prior to initiation of therapy and at least weekly during therapy; monitor for liver function abnormalities by measuring AST and ALT during therapy; perform urinalysis, BUN, and creatinine determinations during therapy, and adjust dose if these values become elevated; if renal impairment is known or suspected, adjust dose and monitor blood levels; these measures avoid possible neurotoxic reactions
Imipenem and cilastatin (Primaxin)
Carbapenem with activity against most gram-positive organisms (except MRSA), gram-negative organisms, and anaerobes. Used for treatment of multiple organism infections in which other agents do not have wide spectrum coverage or are contraindicated due to their potential for toxicity.
Dosing
Adult
500 mg IV q6h
Pediatric
<12 years: Not established
>12 years: Administer as in adults
Interactions
Coadministration with cyclosporine may increase CNS adverse effects of both agents; coadministration with ganciclovir may result in generalized seizures
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Adjust dose in renal insufficiency; avoid use in children <12 years
Meropenem (Merrem)
Carbapenem with slightly increased activity against gram-negative organisms and slightly decreased activity against staphylococci and streptococci compared to imipenem.
Dosing
Adult
1 g IV q8h
Pediatric
Not established
Interactions
Probenecid may inhibit renal excretion of meropenem, increasing meropenem levels
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Pseudomembranous colitis and thrombocytopenia may occur, requiring immediate discontinuation of medication
Clindamycin (Cleocin)
Primarily used for its activity against anaerobes. Has some activity against streptococcus and methicillin-sensitive S aureus (MSSA).
Dosing
Adult
600-900 mg IV q8h
Pediatric
5-10 mg/kg IV q8h
Interactions
Increases duration of neuromuscular blockade induced by tubocurarine and pancuronium
Contraindications
Documented hypersensitivity; regional enteritis; ulcerative colitis; hepatic impairment; antibiotic-associated colitis
Precautions
Pregnancy
D - Fetal risk shown in humans; use only if benefits outweigh risk to fetus
Precautions
Dose adjustment may be necessary in severe hepatic dysfunction; no adjustment is necessary in renal insufficiency; associated with severe and possibly fatal colitis
Metronidazole (Flagyl)
Imidazole ring-based antibiotic active against various anaerobic bacteria and protozoa. Usually employed in combination with other antimicrobial agents, except when it is used for Clostridium difficile enterocolitis in which monotherapy is appropriate.
Dosing
Adult
Loading dose: Infuse 15 mg/kg IV over 1 h or 1 g for a 70-kg adult
Maintenance dose: Infuse 7.5 mg/kg IV over 1 h q6-8h or 500 mg for a 70-kg adult; administer 6 h following the loading dose; not to exceed 4 g in 24 h
Pediatric
Not established
Interactions
Potentiates anticoagulant effect of warfarin; agents that alter hepatic P450 system affect its clearance; phenytoin and phenobarbital may decrease half-life; may reduce metronidazole clearance and increase toxicity; may increase effect of anticoagulants; may decrease lithium and phenytoin clearance, increasing their toxicity; disulfiramlike reactions may occur when used concurrently with orally ingested ethanol (although the risk for most patients may be slight, exercise caution)
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Adjust dose in patients with severe hepatic disease because they may metabolize metronidazole slowly; monitor patients for seizures and the development of peripheral neuropathy
Ciprofloxacin (Cipro)
Fluoroquinolone that inhibits bacterial DNA synthesis and, consequently, growth, by inhibiting DNA gyrase and topoisomerases, which are required for replication, transcription, and translation of genetic material. Quinolones have broad activity against gram-positive and gram-negative aerobic organisms. Has no activity against anaerobes. Continue treatment for at least 2 d (7-14 d typical) after signs and symptoms have disappeared.
Dosing
Adult
400 mg IV q12h
Pediatric
10-15 mg/kg IV q12h
Interactions
Antacids, iron salts, and zinc salts may reduce serum levels; administer antacids 2-4 h before or after taking fluoroquinolones; cimetidine may interfere with metabolism of fluoroquinolones; ciprofloxacin reduces therapeutic effects of phenytoin; probenecid may increase ciprofloxacin serum concentrations
May increase toxicity of theophylline, caffeine, cyclosporine, and digoxin (monitor digoxin levels); may increase effects of anticoagulants (monitor PT)
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Dosage adjustments (adult adjustments)
CrCl (mL/min) <10: 50% of PO or IV dose q12h
HD: 0.25-0.5 g PO or 0.2-0.4 g IV q12h
During peritoneal dialysis: 0.25-0.5 g PO or 0.2-0.4 g IV q8h
In prolonged therapy, perform periodic evaluations of organ system functions (eg, renal, hepatic, hematopoietic); adjust dose in renal function impairment; superinfections may occur with prolonged or repeated antibiotic therapy
Not drug of first choice in pediatrics due to increased incidence of adverse events compared to controls, including arthropathy; no data exist for dose for pediatric patients with renal impairment (ie, CrCl <50 mL/min)
Fluoroquinolones are associated with increased risk of tendinitis and tendon rupture in all ages, this risk is further increased in older patients usually over 60 y, in patients taking corticosteroid drugs, and in patients with kidney, heart, or lung transplants
Activated protein C analogs
Exert antithrombic effects, have indirect profibrinolytic activity, and may have anti-inflammatory effect.
Drotrecogin alfa (Xigris)
Indicated for reduction of mortality in patients with severe sepsis associated with acute organ dysfunction and at high risk of death. Recombinant form of human activated protein C that exerts antithrombotic effect by inhibiting factors Va and VIIIa. Has indirect profibrinolytic activity by inhibiting plasminogen activator inhibitor-1 (PAI-1) and limiting formation of activated thrombin-activatable-fibrinolysis-inhibitor. May exert anti-inflammatory effect by inhibiting human tumor necrosis factor (TNF) production by monocytes, blocking leukocyte adhesion to selectins, and limiting thrombin-induced inflammatory responses within microvascular endothelium.
Dosing
Adult
24 mcg/kg/h IV continuous infusion for 96 h; ideally, initiate within 48 h of sepsis onset
Pediatric
Not established
Interactions
None reported; coadministration with drugs that affect hemostasis may increase risk of bleeding (eg, warfarin, heparin, thrombolytics, glycoprotein IIb/IIIa inhibitors)
Contraindications
Documented hypersensitivity; increased risk of bleeding (eg, active internal bleeding, recent hemorrhagic stroke, recent intraspinal or intracranial surgery, recent or current trauma, presence of epidural catheter, intracranial neoplasm, cerebral herniation, severe head trauma)
Precautions
Pregnancy
C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Bleeding is most common serious adverse effect; caution with conditions that increase risk of bleeding including INR >3, concurrent therapeutic heparin (>15 U/kg/h), within 6 wk of GI bleeding episode, within 3 d of thrombolytic therapy, within 7 d of platelet inhibitors administration, within 3 mo of ischemic stroke, intracranial arteriovenous malformation or aneurysm, known bleeding diathesis, chronic severe hepatic disease; stop infusion if clinically significant bleeding occurs
Vasopressor supportive therapy
If patient does not respond to several liters of isotonic crystalloid (usually 4 or more) or evidence of volume overload is present, the depressed cardiovascular system can be stimulated by inotropic and vasoconstrictive agents.
Dopamine (Inotropin)
Used to treat hypotension in fluid-resuscitated patients. Stimulates both adrenergic and dopaminergic receptors. Hemodynamic effect depends on the dose. Lower doses stimulate mainly dopaminergic receptors that produce renal and mesenteric vasodilation in health volunteers, but probably have no measurable effect in patients who are critically ill. Higher doses produce cardiac stimulation, tachycardia, and vasoconstriction.
Dosing
Adult
In hypotensive hyperdynamic shock: starting dose of 2-5 mcg/kg/min IV; titrate as needed to maintain a MAP >60 mm Hg; may be increased by 1-4 mcg/kg/min q10-30min IV until satisfactory response; not to exceed 20 mcg/kg/min
Maintenance dose: <20 mcg/kg/min IV usually are adequate for 50% of patients treated
Pediatric
Not established
Interactions
Phenytoin, alpha-adrenergic and beta-adrenergic blockers, general anesthesia, and MAO inhibitors increase and prolong the effects
Contraindications
Documented hypersensitivity; tachycardia; pheochromocytoma; ventricular tachyarrhythmias
Precautions
Pregnancy
C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Monitor urine flow, cardiac output, pulmonary wedge pressure, and BP closely during the infusion; prior to infusion, correct hypovolemia with either whole blood or plasma, as indicated; monitoring of central venous pressure or left ventricular filling pressure may be helpful in detecting and treating hypovolemia
Norepinephrine (Levophed)
As with dopamine, it is used to treat hypotension following adequate fluid-volume replacement. Norepinephrine stimulates beta 1-adrenergic and alpha-adrenergic receptors, which increase arterial tone and cardiac contractility. As a result, systemic BP and coronary blood flow increases with norepinephrine, though myocardial oxygen demand also may increase. After obtaining a response, adjust infusion rate to maintain a MAP greater than 60 mm Hg. BP levels below this threshold are insufficient to perfuse vital organs while increasing pressures much greater than 70 mm Hg, using vasopressors does not further increase tissue blood flow.
Dosing
Adult
0.05-2 mcg/kg/min IV; titrate to effect
Pediatric
Not established
Interactions
Atropine sulfate may enhance pressor response of norepinephrine by blocking reflex bradycardia caused by norepinephrine
Contraindications
Documented hypersensitivity; known hypovolemia; peripheral or mesenteric vascular thrombosis because ischemia may be increased and the area of the infarct extended
Precautions
Pregnancy
D - Fetal risk shown in humans; use only if benefits outweigh risk to fetus
Precautions
Correct blood-volume depletion, if possible, before administering norepinephrine therapy; extravasation may cause severe tissue necrosis, administer into a large vein; use with caution in patients with occlusive vascular disease
Vasopressin (Pitressin)
Has vasopressor and antidiuretic hormone (ADH) activity. Although vasopressin does not increase BP in healthy subjects, it markedly increases vasomotor tone in patients with septic shock. It also increases water resorption at the distal renal tubular epithelium (ADH effect) and promotes smooth muscle contraction throughout the vascular bed of the renal tubular epithelium (vasopressor effects). Vasoconstriction also is increased in splanchnic, portal, coronary, cerebral, peripheral, pulmonary, and intrahepatic vessels. Vasopressin is not yet routinely used to treat hypotension in septic shock. The dosage of vasopressin used for hypotension is one-tenth that used to treat upper GI bleeding from varices.
Dosing
Adult
Suggested dose: 0.01-0.05 U/min IV; titrate dose as needed
Pediatric
Not established
Interactions
Lithium, epinephrine, demeclocycline, heparin, and alcohol may decrease the effects of vasopressin; conversely, chlorpropamide, urea, fludrocortisone, and carbamazepine potentiate its effects
Contraindications
Documented hypersensitivity; coronary artery disease
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Caution in cardiovascular disease, seizure disorders, nitrogen retention, asthma, or migraine; excessive doses may result in hyponatremia
If patients are treated initially in the wards or in the emergency department, after initial attempts at stabilization, transfer them to the ICU for invasive monitoring and support.
For excellent patient education resources, visit eMedicine's Blood and Lymphatic System Center. Also, see eMedicine's patient education article Sepsis (Blood Infection).
In 1914, Schottmueller wrote, "Septicemia is a state of microbial invasion from a portal of entry into the blood stream which causes sign of illness." The definition did not change significantly over the years because sepsis and septicemia were considered to refer to a number of ill-defined clinical conditions in addition to bacteriemia. In practice, the terms were often used interchangeably; however, less than one half of the patients who have signs and symptoms of sepsis have positive blood culture results.
In the late 1960s, several reports appeared describing remote organ failure (eg, pulmonary failure, liver failure) as a complication of severe sepsis. In 1975, a classic editorial by Baue was entitled "Multiple, progressive or sequential systems failure, a syndrome of the 1970s." This concept was formulated as the basis of a new clinical syndrome. Several terms were cloned thereafter, such as multiple organ failure, multiple system organ failure, and multiple organ system failure, to describe this evolving clinical syndrome of otherwise unexplained progressive physiological failure of several interdependent organ systems. More recently, the term multiple organ dysfunction syndrome (MODS) has been proposed as a more appropriate description.
Multiorgan failure from sepsis
Sepsis is a clinical syndrome that complicates severe infection and is characterized by systemic inflammation and widespread tissue injury. Multiple organ dysfunction is a continuum, with incremental degrees of physiological derangements in individual organs; it is a process rather than an event. Alteration in organ function can vary widely from a mild degree of organ dysfunction to completely irreversible organ failure. The degree of organ dysfunction has a major clinical impact. The term MODS is defined as a clinical syndrome in which the development of progressive and potentially reversible physiological dysfunction in 2 or more organs or organ systems induced by a variety of acute insults, including sepsis, is characteristic.The American College of Chest Physicians/Society of Critical Care Medicine Consensus Panel developed definitions of the various stages of sepsis, which can be summarized as follows:
- Infection is a microbial phenomenon in which an inflammatory response to the presence of microorganisms or the invasion of normally sterile host tissue by these organisms is characteristic.
- Bacteremia is the presence of viable bacteria in the blood.
- Systemic inflammatory response syndrome (SIRS) may follow a variety of clinical insults, including infection, pancreatitis, ischemia, multiple trauma, tissue injury, hemorrhagic shock, or immune-mediated organ injury.
- Sepsis is a systemic response to infection. This is identical to SIRS, except that it must result from infection.
- Septic shock is sepsis with hypotension (systolic BP <90 mm Hg or a reduction of 40 mm Hg from baseline) despite adequate fluid resuscitation. Concomitant organ dysfunction or perfusion abnormalities (eg, lactic acidosis, oliguria, coma) are present in the absence of other known causes.
- MODS is the presence of altered organ function in a patient who is acutely ill such that homeostasis cannot be maintained without intervention. Primary MODS is the direct result of a well-defined insult in which organ dysfunction occurs early and can be directly attributable to the insult itself. Secondary MODS develops as a consequence of a host response and is identified within the context of SIRS. The inflammatory response of the body to toxins and other components of microorganisms causes the clinical manifestations of sepsis.
- Temperature greater than 38°C or less than 36°C
- Heart rate greater than 90 beats per minute
- Respiratory rate greater than 20 breaths per minute or a PaCO2 in arterial gas less than 32 mm Hg
- WBC count greater than 12,000 cells/µL, less than 4000 cells/µL, or greater than 10% band forms
Pathophysiology
Sepsis has been referred to as a process of malignant intravascular inflammation. Normally, a potent, complex, immunologic cascade ensures a prompt protective response to microorganism invasion in humans. A deficient immunologic defense may allow infection to become established; however, an excessive or poorly regulated response may harm the host through maladaptive release of indigenously generated inflammatory compounds.
Lipid A and other bacterial products release cytokines and other immune modulators that mediate the clinical manifestations of sepsis. Interleukins, tumor necrosis factor-alpha (TNF-alpha), interferon gamma, and other colony-stimulating factors are produced rapidly within minutes or hours after interactions of monocytes and macrophages with lipid A. Inflammatory mediators release becomes a self-stimulating process (an autocrine), and release of other inflammatory mediators, including interleukin-1 (IL-1), platelet activating factor, IL-2, IL-6, IL-8, IL-10, INF, and nitric oxide, further increases cytokine levels. This leads to continued activation of polymorphonuclear leukocytes (PMNs), macrophages, and lymphocytes; proinflammatory mediators recruit more of these cells (a paracrine process). All of these processes create a state of destructive immunologic dissonance.
Sepsis is described as an autodestructive process that permits extension of the normal pathophysiologic response to infection to involve otherwise normal tissues and results in MODS.
Specific organ involvement
Organ dysfunction or organ failure may be the first clinical sign of sepsis, and no organ system is immune from the consequences of the inflammatory excesses of sepsis. Mortality rates increase with the increase of failed organs.
 Circulation
Circulation
Significant derangement in autoregulation of circulation is typical of sepsis. Vasoactive mediators cause vasodilatation and increase the microvascular permeability at the site of infection. Nitric oxide plays a central role in the vasodilatation of septic shock. Also, impaired secretion of vasopressin may occur, which may permit persistence of vasodilatation.Central circulation: Changes in both systolic and diastolic ventricular performance occur in sepsis. Through the use of the Frank Starling mechanism, cardiac output often is increased to maintain the BP in the presence of systemic vasodilatation. Patients with preexisting cardiac disease are unable to increase their cardiac output appropriately.
Regional circulation: Sepsis interferes with the normal distribution of systemic blood flow to organ systems; therefore, core organs may not receive appropriate oxygen delivery leading to what is knows as regional hypoperfusion.
Microcirculation is the key target organ for injury in sepsis syndrome. A decrease in the number of functional capillaries causes an inability to extract oxygen maximally, which is caused by intrinsic and extrinsic compression of capillaries and plugging of the capillary lumen by blood cells. Increased endothelial permeability leads to widespread tissue edema of protein-rich fluid.
Septic shock and the systemic inflammatory response is characterized by reversible myocardial depression, which can prove resistant to catecholamine and fluid administration. Circulating "myocardial depressant factor"—probably representing the synergistic effects of TNFα, IL-1β, other cytokines, and nitric oxide—is implicated in pathogenesis. Macrovascular myocardial ischemia and hypoperfusion are unlikely contributors. In severe sepsis and septic shock, microcirculatory dysfunction, and mitochondrial depression cause regional tissue distress, therefore, regional hypoxia persists. This condition is termed microcirculatory and mitochondrial distress syndrome (MMDS).[1 ]Sepsis-induced inflammatory autoregulatory dysfunction persists and oxygen need is not matched by supply, leading to multiorgan system dysfunction.
Redistribution of intravascular fluid volume resulting from reduced arterial vascular tone, diminished venous return from venous dilation, and release of myocardial depressant substances causes hypotension.
Pulmonary dysfunction
Endothelial injury in the pulmonary vasculature leads to disturbed capillary blood flow and enhanced microvascular permeability, resulting in interstitial and alveolar edema. Neutrophil entrapment within the pulmonary microcirculation initiates and amplifies the injury to alveolar capillary membranes. Acute respiratory distress syndrome (ARDS) is a frequent manifestation of these effects.Gastrointestinal dysfunction
The GI tract may help propagate the injury of sepsis. Overgrowth of bacteria in the upper GI tract may be aspirated into the lungs, producing nosocomial or aspiration pneumonia. The normal barrier function of the gut may be affected, allowing translocation of bacteria and endotoxins into the systemic circulation and extending the septic response. Septic shock can cause paralytic ileus that can lead to a delay in institution of enteral feeding. The optimal level of nutritional intake is interfered with in the face of high protein and calorie requirements. Narcotics and muscle relaxants can further worsen the gastrointestinal tract motility.Liver
By virtue of the role of the liver in host defense, the abnormal synthetic functions caused by liver dysfunction can contribute to both the initiation and progression of sepsis. The reticuloendothelial system of the liver acts as a first line of defense in clearing bacteria and their products; liver dysfunction leads to a spillover of these products into systemic circulation.Liver failure or "shock liver" can manifest by elevation of liver enzymes and bilirubins, coagulation defects, and failure to excrete toxins such as ammonia, which lead to worsening encephalopathy.
Renal dysfunction
Acute renal failure often accompanies sepsis due to acute tubular necrosis. The mechanism is complex but involve decrease effective intravascular volume due to systemic hypotension, direct renal vasoconstriction, release of cytokines, and activation of neutrophils by endotoxins and other peptides, which contribute to renal injury.Central nervous system dysfunction
Involvement of the CNS in sepsis produces encephalopathy and peripheral neuropathy. The pathogeneses is poorly defined but likely related to systemic hypotension, which can lead to brain hypoperfusion.Coagulopathy
Subclinical coagulopathy signified by a mild elevation of the thrombin or activated partial thromboplastin time (aPTT) or a moderate reduction in platelet count is extremely common, but overt disseminated intravascular coagulation (DIC) could happen. Deficiencies of coagulation system proteins, including protein C, antithrombin III, and tissue factor inhibitors, cause coagulopathy.Mechanisms of organ dysfunction and injury
The precise mechanisms of cell injury and resulting organ dysfunction in sepsis are not understood fully. Multiorgan dysfunction syndrome is associated with widespread endothelial and parenchymal cell injury, some of which can be explained by the following proposed mechanisms:- Hypoxic hypoxia: The septic circulatory lesion disrupts tissue oxygenation, alters the metabolic regulation of tissue oxygen delivery, and contributes to organ dysfunction. Microvascular and endothelial abnormalities contribute to the septic microcirculatory defect in sepsis. The reactive oxygen sepsis, lytic enzymes, and vasoactive substances (nitric oxide, endothelial growth factors) lead to microcirculatory injury, which is compounded by the inability of the erythrocytes to navigate the septic microcirculation.
- Direct cytotoxicity: The endotoxin, TNF-alpha, and nitric oxide may cause damage to mitochondrial electron transport, leading to disordered energy metabolism. This is called cytopathic or histotoxic anoxia, an inability to utilize oxygen even when it is present.
- Apoptosis: Apoptosis (programmed cell death) is the principal mechanism by which dysfunctional cells are eliminated normally. The proinflammatory cytokines may delay apoptosis in activated macrophages and neutrophils, but other tissues, such as the gut epithelium, may undergo accelerated apoptosis. Therefore, derangement of apoptosis plays a critical role in tissue injury of sepsis.
- Immunosuppression: The interaction between proinflammatory and anti-inflammatory mediators may lead to an imbalance. An inflammatory reaction or immunodeficiency may predominate, or both may be present.
Characteristics of sepsis that influence outcomes
Clinical characteristics that relate to the severity of sepsis include an abnormal host response to infection, the site and type of infection, the timing and type of antimicrobial therapy, the offending organism, and the development of shock, underlying disease, the patients' chronic health condition, and the number of failed organs. Factors that lead to sepsis and septic shock may not be essential in determining the ultimate outcome.Epidemiology
Estimating the exact incidence of sepsis in the world is difficult. Studies vary in their methods of determining the incidence of sepsis.Clinical
History
Symptoms of sepsis are usually nonspecific and include fever, chills, and constitutional symptoms of fatigue, malaise, anxiety, or confusion.[4 ]These symptoms are not pathognomonic for infection and may be observed in a wide variety of noninfectious inflammatory conditions. They may be absent in serious infections, especially in elderly individuals.
- Sepsis, SIRS, septic shock, and multiorgan dysfunction syndrome represent a clinical continuum. The specific features exhibited depend on where the patient's case falls on that continuum. SIRS is defined by the presence of 2 or more of the following:
- Temperature greater than 38.0°C or less than 36.0°C
- Heart rate greater than 90 beats per minute
- Respiratory rate greater than 20 breaths per minute
- WBC count greater than 12,000 cells/µL, less than 4000 cells/µL, or more than 10% bands
- Fever is a common feature of sepsis. Fever from an infectious etiology results from resetting the hypothalamus so that heat production and heat loss are balanced to maintain a higher temperature. An abrupt onset of fever usually is associated with a large infectious load.
- Chills are a secondary symptom associated with fever and result from increased muscular activity in an attempt to produce heat in order to raise the body temperature to the level required to reset the hypothalamus.
- Sweating occurs when the hypothalamus returns to its normal set point and senses that the body temperature is above the desired level. Perspiration is stimulated to evaporate and cool excess body heat.
- Alteration in mental function often is observed. Mild disorientation or confusion especially is common in elderly individuals. More severe manifestations include apprehension, anxiety, and agitation, and it may eventually lead to coma. The mechanism of alteration in mental function is not known, but altered amino acid metabolism has been proposed as one cause of metabolic encephalopathy.
- Hyperventilation with respiratory alkalosis is a common feature of sepsis. Stimulation of the medullary ventilatory center by endotoxins and other inflammatory mediators has been proposed as the cause of hyperventilation.
- The following localizing symptoms are some of the most useful clues to the etiology of both fever and sepsis:
- Head and neck infections - Earache, sore throat, sinus pain, or swollen lymph glands
- Chest and pulmonary infections - Cough, especially if productive; pleuritic chest pain; and dyspnea
- Abdominal and GI infections - Abdominal pain, nausea, vomiting, and diarrhea
- Pelvic and genitourinary infections - Pelvic or flank pain, vaginal or urethral discharge, urea, frequency, urgency
- Bone and soft tissue infections - Focal pain or tenderness, focal erythema, edema
Physical
Physical examination notes the general condition of the patient first. Observe the overall hemodynamic condition to search for signs of hyperperfusion. Look for signs suggestive of a focal infection. An acutely ill, toxic appearance is a common feature in serious infections.
- The vital signs may suggest sepsis, even if fever is absent. As noted above, tachypnea is common; tachycardia with an increased pulse pressure also is common.
- Measure the body temperature accurately. Oral temperatures often are unreliable; obtain rectal temperatures.
- Investigate signs of systemic tissue perfusion. In the early stages of sepsis, cardiac output is well maintained or even increased. Along with vasodilatory mediators, this may result in warm skin, warm extremities, and normal capillary refill. As sepsis progresses, stroke volume and cardiac output fall. Patients begin to manifest signs of poor distal perfusion, including cool skin, cool extremities, and delayed capillary refill.
- The following physical signs suggest focal, usually bacterial, infection:
- CNS infection - Profound depression in mental status and meningismus
- Head and neck infections - Inflamed or swollen tympanic membranes, sinus tenderness, pharyngeal exudates, stridor, cervical lymphadenopathy
- Chest and pulmonary infections - Localized rales or evidence of consolidation
- Cardiac infections - Regurgitant valvular murmur
- Abdominal and GI infections - Focal tenderness, guarding or rebound, rectal tenderness, or swelling
- Pelvic and genitourinary infections - Costovertebral angle tenderness, pelvic tenderness, cervical motion pain, and adnexal tenderness
- Bone and soft tissue infections - Focal erythema, edema, infusion, and tenderness
- Skin infections - Petechiae and purpura
Differential Diagnoses
- Acute Renal Failure
- Shock, Distributive
- Acute Respiratory Distress Syndrome
- Shock, Hemorrhagic
- Cardiogenic Shock
- Streptococcus Group A Infections
- Infective Endocarditis
- Systemic Inflammatory Response Syndrome
- Pneumococcal Infections
- Toxic Shock Syndrome
- Pneumonia, Bacterial
- Urinary Tract Infection, Females
- Sepsis, Bacterial
- Urinary Tract Infection, Males
- Septic Shock
- Ventilation, Mechanical
Workup
Laboratory Studies
- Laboratory tests are useful in suspected sepsis or septic shock to assess the general hematologic and metabolic condition of the patient. The microbiologic studies provide results, which may indicate occult bacterial infection or bacteremia, and indicate the specific microbial etiology.
- CBC count with differential: An adequate hemoglobin concentration is necessary to ensure oxygen delivery in shock. Maintain the hemoglobin at a level of 8 g/dL.
- Platelets: Acute phase reactants, platelets usually increase at the onset of any serious stress. The platelet count will fall with persistent sepsis, and DIC may develop.
- WBC count: The white cell differential and the WBC count may predict the existence of a bacterial infection. In adults who are febrile, a WBC count greater than 15,000 cells/µL or a neutrophil band count greater than 1500 cells/µL is associated with a high likelihood of bacterial infection.[5 ]
- Metabolic assessment: Perform metabolic assessment with serum electrolytes, including magnesium, calcium, phosphate, and glucose, at regular intervals.
- Renal and hepatic function: Assess renal and hepatic function with serum creatinine, BUN, bilirubin, alkaline phosphate, and alanine aminotransferase (ALT).
- ABG
- Measurement of serum lactate provides an assessment of tissue hypoperfusion.
- Elevated serum lactate indicates that significant tissue hypoperfusion exists with the shift from aerobic to anaerobic metabolism.
- Serum lactate: Higher serum lactate indicates a worse degree of shock and a higher mortality.
- Prothrombin time (PT) and activated partial thromboplastin time (aPTT): Assess coagulation status with prothrombin time (PT) and activated partial thromboplastin time (aPTT). Patients with clinical evidence of coagulopathy require additional tests to detect the presence of DIC.
- Blood cultures: Indiscriminate use of blood cultures has low utility.
- Blood culture is the primary modality for aiding in the diagnosis for intravascular infections (eg, endocarditis) and infections of indwelling intravascular devices.
- Two populations, people who abuse IV drugs and patients with prosthetic heart valves, are at high risk for endocarditis.
- Patients at risk for bacteremia include adults who are febrile with an elevated WBC or neutrophil band counts, elderly patients who are febrile, and patients who are febrile and neutropenic. These populations have a 20-30% incidence of bacteremia.
- The incidence of bacteremia is at least 50% in patients with sepsis and evidence of end-organ dysfunction.
- Urinalysis and urine culture: Order a urinalysis and urine culture for every patient who is septic. Urinary infection is a common source for sepsis, especially in elderly individuals. Adults who are febrile without localizing symptoms or signs have a 10-15% incidence of occult urinary tract infection (UTI).
- Tissue staining and culture: Obtain secretions or tissue for Gram stain and culture from sites of potential infection. Generally, the Gram stain is the only available test to immediately document the presence of bacterial infection and guide the choice of initial antibiotic therapy.
Imaging Studies
- A variety of imaging modalities are employed to diagnose clinically suspected focal infection, detect the presence of a clinically occult focal infection, and detect complications of sepsis and septic shock.
- Obtain a chest radiograph in patients with severe sepsis because the clinical examination is unreliable for pneumonia. Clinically occult infiltrates have been detected by routine use of chest radiography in adults who are febrile without localizing symptoms or signs and in patients who are febrile and neutropenic without pulmonary symptoms.
- Supine and upright or lateral decubitus abdominal films may be useful when an intraabdominal source is suspected.
- Ultrasound is the imaging modality of choice when a biliary tract source is suspected to be the source of sepsis.
- CT scan is the imaging modality of choice for excluding intraabdominal abscess or a retroperitoneal source of infection.
- Obtain a CT scan of the head in patients with evidence of increased intracranial pressure (papilledema) or suggestion of focal mass lesions (eg, focal defects, previous sinusitis or otitis, recent intracranial surgery) or prior to lumbar puncture (LP) when meningitis is suspected.
- When clinical evidence of a deep, soft tissue infection exists, such as, crepitus, bullae, hemorrhage, or foul smelling exudate, obtain a plain radiograph. The presence of soft tissue gas and the spread of infection beyond clinically detectable disease may require surgical exploration.
Procedures
- The LP needs to be performed urgently when meningitis or encephalitis is suspected. In patients with an acute fulminant presentation, rapid onset of septic shock, and severe impairment of mental status, rule out bacterial meningitis by LP.
- Procedures, such as cardiac monitoring, noninvasive BP monitoring, and pulse oximetry, are necessary because patients often require ICU admission for invasive monitoring and support.
- Supplemental oxygen is provided during initial stabilization and resuscitation.
- All patients in septic shock should have adequate venous access for volume resuscitation. A central venous line also can be used to monitor central venous pressure to assess intravascular volume status.
- An indwelling urinary catheter used to monitor urinary output is used as a marker for adequate renal perfusion and cardiac output.
- Patients who have developed septic shock require right heart catheterization with a pulmonary artery (Swan-Ganz) catheter. This catheter provides an accurate assessment of the volume status of a patient who is septic. The cardiac output measurement can be obtained. Furthermore, determination of mixed venous oxygenation is helpful in determining the status of tissue oxygenation.
- Most patients who are septic develop respiratory distress secondary to severe sepsis or as a manifestation of septic shock. Pulmonary dysfunction of sepsis (ARDS) also may occur. These patients need intubation and mechanical ventilation for optimum respiratory support.
Staging
Two well-defined forms of multiorgan dysfunction syndrome exist. In both, the development of acute lung injury or ARDS is of key importance to the natural history. ARDS is the earliest manifestation in all cases.
- In the more common form of multiorgan dysfunction syndrome, the lungs are the predominant, and often the only, organ system affected until very late in the disease. These patients most often present with primary pulmonary disorder, such as pneumonia, aspiration, contusion, near drowning, exacerbation of chronic obstructive pulmonary disease (COPD), hemorrhage, or pulmonary embolism. Lung disease progresses to meet ARDS criteria. Encephalopathy or mild coagulopathy may accompany pulmonary dysfunction, which persists for 2-3 weeks. At this time, the patient either begins to recover or progresses to develop fulminant dysfunction in another organ system. Once another major organ dysfunction occurs, these patients frequently do not survive.
- The second form of multiorgan dysfunction syndrome presents quite differently. These patients often have an inciting source of sepsis in organs other than the lungs, the most common being intraabdominal sepsis, extensive blood loss, pancreatitis, or vascular catastrophes. Acute lung injury or ARDS develops early, and dysfunction in other organ systems also develops much sooner than in the more common form of multiorgan dysfunction syndrome. The organ systems affected are hepatic, hematological, cardiovascular, and renal. Patients remain in a pattern of compensated dysfunction for several weeks, at which time they either recover or deteriorate further and die.
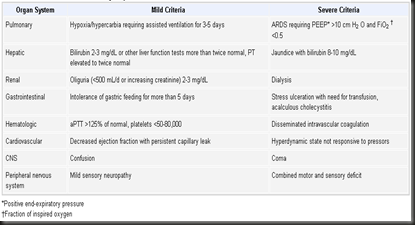
Criteria for Organ Dysfunction(click the pic to enlarge)

Treatment
Medical Care
The treatment of patients with septic shock consists of the following 3 major goals: (1) Resuscitate the patient from septic shock using supportive measures to correct hypoxia, hypotension, and impaired tissue oxygenation. (2) Identify the source of infection and treat with antimicrobial therapy, surgery, or both. (3) Maintain adequate organ system function guided by cardiovascular monitoring and interrupt the pathogenesis of multiorgan system dysfunction.
The principles in the management of septic shock based on current literature include the following components:
- Early recognition
- Early and adequate antibiotic therapy
- Source control
- Early hemodynamic resuscitation and continued support
- Corticosteroids (refractory vasopressor-dependent shock)
- Drotrecogin alfa (severely ill if APACHE II score >25)
- Tight glycemic control
- Proper ventilator management with low tidal volume in patients with ARDS
- General supportive care
- Initial treatment includes support of respiratory and circulatory function, supplemental oxygen, mechanical ventilation, and volume infusion. Treatment beyond these supportive measures includes a combination of several parenteral antibiotics, removal or drainage of infected foci, treatment of complications, and pharmacologic interventions to prevent further harmful host responses.
- Administer supplemental oxygen to any patient who is septic with hypoxia or respiratory distress. If the patient's airway is not secure or respirations are inadequate, perform endotracheal intubation and mechanical ventilation.
- Intravascular volume resuscitation
- All patients with sepsis require supplemental fluids. Assessment of the patient's volume and cardiovascular status guides the amount and rate of infusion. For adult patients who are hypotensive, administer an isotonic crystalloid solution (sodium chloride 0.9% or Ringer lactate) in boluses of 500 mL (10 mL/kg in children), with repeat clinical assessments after each bolus. Administer repeat boluses until signs of adequate perfusion are restored. A total of 4-6 L may be required. Monitor patients for signs of volume overload, such as dyspnea, pulmonary crackles, and pulmonary edema, on chest radiograph. Improvement, stabilization, and normalization of the patient's mental status, heart rate, BP, capillary refill, and urine output indicate adequate volume resuscitation.
- In some patients, clinically assessing the response to volume infusion may be difficult. By monitoring the response of central venous pressure or pulmonary artery occlusion pressure (PAOP) to fluid boluses, the physician can assess these patients. A control venous pressure of 10-15 mm Hg, a PAOP greater than 18 mm Hg, or a rise in the PAOP by 5 mm Hg or more following fluid bolus indicates adequate volume resuscitation. Such patients are susceptible to volume overload; therefore, administer further fluid carefully. Colloid resuscitation (with albumin or pentastarch) has no proven benefit over isotonic crystalloid resuscitation (normal saline or Ringer lactate).
- Empirical antimicrobial therapy
- Administer initial antibiotics. Selection of particular agents is empirical and is based on an assessment of the patient's underlying host defenses, the potential sources of infection, and the most likely responsible organisms. Antibiotics must be broad spectrum and cover gram-positive, gram-negative, and anaerobic bacteria because all classes of these organisms produce identical clinical pictures. Administer antibiotics parenterally in doses adequate to achieve bactericidal serum levels. Many studies have found that clinical improvement correlates with the achievement of serum bactericidal levels rather than the number of antibiotics administered.
- Include coverage directed against anaerobes in the therapy of patients with intraabdominal or perineal infections. Antipseudomonal coverage is indicated in patients with neutropenia or burns. Patients who are immunocompetent usually can be treated with a single drug with broad-spectrum coverage, such as a third-generation cephalosporin. Patients who are immunocompromised usually require dual antibiotic coverage with broad-spectrum antibiotics with overlapping coverage. Within these general guidelines, no single combination of antibiotics is clearly superior to others.
- Vasopressor supportive therapy
- When proper fluid resuscitation fails to restore hemodynamic stability and tissue perfusion, initiate therapy with vasopressor agents. These agents are dopamine, norepinephrine, epinephrine, and phenylephrine. These vasoconstricting drugs maintain adequate BP during life-threatening hypotension and preserve perfusion pressure for optimizing flow in various organs. Maintain the mean BP required for adequate splanchnic and renal perfusion (mean arterial pressure [MAP] of 60 or 65 mm Hg) based on clinical indices for organ perfusion.
- If the patient remains hypotensive despite volume infusion and moderate dose dopamine, start a direct vasoconstrictor (eg, norepinephrine) at a dose of 0.5 mcg/kg/min in and titrated to support a systolic BP of 90 mm Hg. Although potent vasoconstrictors (eg, norepinephrine) traditionally have been avoided because of their adverse events on cardiac output and renal perfusion, human data has shown that norepinephrine can reverse septic shock in patients unresponsive to volume and dopamine. These patients require invasive hemodynamic monitoring with arterial lines and pulmonary artery catheters. A brief discussion of the hemodynamic drugs used to support patients who are critically ill and septic follows:
- Vasopressor therapy
- Dopamine: A precursor of norepinephrine and epinephrine, dopamine has varying effects based on the doses. A dose of less than 5 mcg/kg/min results in vasodilation of renal, mesenteric, and coronary beds. At a dose of 5-10 mcg/kg/min, beta-1-adrenergic effects induce an increase in cardiac contractility and heart rate. At doses about 10 mcg/kg/min, alpha-adrenergic effects lead to arterial vasoconstriction and an increase in BP. Dopamine is effective in increasing MAP in patients who are hypotensive with septic shock after volume resuscitation. The BP increases primarily as a result of an inotropic effect and, thus, is useful in patients who have concomitant reduced cardiac function. The undesirable effects are tachycardia, increased pulmonary shunting, potential to decrease splanchnic perfusion, and increased pulmonary arterial wedge pressure.
- Epinephrine: Epinephrine can increase MAP by increasing the cardiac index, stroke volume, systemic vascular resistance, and heart rate. Epinephrine may increase oxygen delivery and consumption and decreases splanchnic blood flow. Epinephrine administration is associated with an elevation of systemic and regional lactate concentrations. The use of epinephrine is recommended in patients who are unresponsive to traditional agents. The undesirable effects are an increase in lactate concentration, a potential to produce myocardial ischemia and arrhythmias, and a reduction in splanchnic flow.
- Norepinephrine: Norepinephrine is a potent alpha-adrenergic agonist with minimal beta-adrenergic agonist effects. Norepinephrine can successfully increase BP in patients who are septic and remain hypotensive following fluid resuscitation and dopamine. The dose of norepinephrine may vary from 0.2-1.35 mcg/kg/min; doses as large as 3.3 mcg/kg/min have been used because alpha-receptor down regulation may occur in sepsis. In patients who are septic, indices of regional perfusion, such as urine flow and lactate concentration, have improved following norepinephrine infusion. Two recent trials have shown that a significantly greater proportion of patients treated with norepinephrine were successfully resuscitated as opposed to patients treated with dopamine. Therefore, use norepinephrine early, and do not withhold it as a last resort. The studies have shown no effects on splanchnic oxygen consumption and hepatic glucose production, provided adequate cardiac output is maintained.
- Phenylephrine: Phenylephrine is a selective alpha-1 adrenergic receptor agonist primarily used in anesthesia to increase BP. Although studies are limited, phenylephrine increased the MAP in patients who are septic and hypotensive with an increase in oxygen consumption and potential to reduce cardiac output. Phenylephrine may be a good choice when tachyarrhythmias limit therapy with other vasopressors.
- Inotropic therapy: Although myocardial performance is altered during sepsis and septic shock, cardiac output usually is maintained in the patients who are septic and have been volume resuscitated. Data from the 1980s and 1990s suggested a linear relationship between oxygen delivery and oxygen consumption (pathologic supply dependency), indicating that oxygen delivery was likely insufficient to meet the metabolic needs of the patient. However, recent investigations have challenged the concept of pathologic supply dependency and the practice of elevating cardiac index and oxygen delivery (hyperresuscitation) because these interventions have not been shown to improve patient outcome. Therefore, the role of inotropic therapy is uncertain unless the patient has an inadequate cardiac index, MAP, mixed venous oxygen saturation, and urine output despite optimal volume resuscitation and vasopressor therapy.
- Renal-dose dopamine: The use of renal-dose dopamine in sepsis is a controversial issue. In the past, low-dose dopamine was routinely used in many units because of the presumed renal protective effects. Dopamine at a dose of 2-3 mcg/kg/min is known to initiate diuresis by increasing renal blood flow in healthy animals and volunteers. Multiple studies have not demonstrated a beneficial effect with prophylactic or therapeutic low-dose dopamine administration in patients who are critically ill. Low-dose dopamine does not protect the patient from developing acute renal failure, and there is no data stating that it preserves mesenteric profusion; the routine use of this practice is not recommended. Aggressively resuscitating patients with septic shock, maintaining adequate perfusion pressure, and avoiding excessive vasoconstriction are effective measures to protect the kidneys.
- Recombinant human-activated protein C
- The inflammatory mediators are known to cause activation of coagulation inhibitors of fibrinolysis, thereby causing diffuse endovascular injury, multiorgan dysfunction, and death. Activated protein C is an endogenous protein that not only promotes fibrinolysis and inhibits thrombosis and inflammation but also may modulate the coagulation and inflammation of severe sepsis. Sepsis reduces the level of protein C and inhibits conversion of protein C to activated protein C. Administration of recombinant activated protein C inhibits thrombosis and inflammation, promotes fibrinolysis, and modulates coagulation and inflammation.
- A study by Jaimes et al was not able to demonstrate beneficial effects of unfractionated heparin in patients with sepsis on length of hospital stay, multiple organ dysfunction, and mortality at 28 days when compared to placebo.[6 ]
- A recent publication by the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group demonstrated that the administration of recombinant human activated protein C (drotrecogin-alpha, activated) resulted in lower mortality rates (24.7% vs 30.8%) in the treated group compared with placebo. Treatment with drotrecogin-alpha, activated was associated with reduction in the relative risk of death by 19.4% (95% CI, 6.6-30.5) and an absolute reduction in risk of death by 6.1%, (P =.005).
- Corticosteroids
- While theoretical and experimental animal evidence exists for the use of large doses of corticosteroids in those with severe sepsis and septic shock, all randomized human studies (except 1 from 1976) found that corticosteroids did not prevent the development of shock, reverse the shock state, or improve the 14-day mortality rate. Therefore, no support exists in the medical literature for the routine use of high doses of corticosteroids in patients with sepsis or septic shock. A meta-analysis of 10 prospective, randomized, controlled trials of glucocorticoid use did not report any benefit from corticosteroids. Therefore, high-dose corticosteroids should not be used in patients with severe sepsis or septic shock.
- Although further studies await further confirmation, current recommendations are as follows:
- Drotrecogin alpha (activated protein C) is the only widely accepted drug specific to the therapy of sepsis. Drotrecogin Alpha should be considered for patients with APACHE II scores greater than 25.
- The main side effect of Drotrecogin alpha is bleeding.
- Recent trials of stress-dose glucocorticoids demonstrated positive results of stress-dose administration of corticosteroids in patients with severe and refractory shock.[7 ]Although further confirmatory studies are awaited, stress-dose steroid coverage should be provided to patients who have the possibility of adrenal suppression.
- The following key points summarize use of corticosteroids in septic shock:
- Older, traditional trials of corticosteroids in sepsis were likely unsuccessful due to high doses and poor patient selection.
- Recent trials with low-dose (physiologic) dosages in select patient populations (vasopressor dependent and possibly relative adrenal insufficiency) patients have resulted in improved outcome.
- Corticosteroids should be initiated for patients with vasopressor-dependent septic shock.
- A cosyntropin stimulation test may be performed to identify patients with relative adrenal insufficiency defined recently as failure to increase levels to more than 9 mcg/dL.
- Tight glycemic control has recently become a prominent emphasis in the care of critically ill patients, and recent data has been extrapolated to potentially apply to septic populations. A 2001 Belgian study of surgical intensive care unit (ICU) patients that remained in the ICU for more than 5 days showed a 10% mortality benefit in those with tighter glycemic control. The glucose levels for these patients were maintained between 80 and 110 mg per deciliter through use of intensive insulin therapy. The benefit of glycemic control appears to result more from aggressive avoidance of the detrimental effects of hyperglycemia rather than the potential therapeutic effect of insulin.
- Based on the current evidence, the Surviving Sepsis Campaign recommends maintaining a glucose level of less than 150 mg/dL.[8 ]
- Tight glycemic control has been shown to improve mortality in postoperative surgical patients including and particularly those patients with sepsis.
- The Surviving Sepsis Campaign recommends that glucose levels in the septic patient should be kept at less than 150 mg/dL.
Surgical Care
Take patients with infected foci to surgery after initial resuscitation and administration of antibiotics for definitive surgical treatment. Little is gained by spending hours stabilizing the patient when an infected focus persists.
Consultations
- Patients who do not respond to therapy or are in septic shock require admission to an ICU for continuous monitoring and observation. Consultation with a critical care physician or internist with expertise is appropriate.
- Seek consultation with an appropriate surgeon for patients with suspected or known infected foci, especially for patients with a suspected abdominal source.
Medication
The proven medical treatments for septic shock are restoration of intravascular volume and broad-spectrum empirical antibiotic coverage. All other medical therapies, while theoretically attractive, have not reduced morbidity or mortality.
Isotonic crystalloids
Standard fluid used for initial volume resuscitation. These fluids expand the intravascular and interstitial fluid spaces. Typically, approximately 30% of administered isotonic fluid remains intravascular; therefore, large quantities may be required to maintain an adequate circulating volume.Normal saline (NS) and Ringer lactate (RL)
Both fluids essentially are isotonic and have equivalent volume restorative properties. While some differences exist between metabolic changes observed with administration of large quantities of either fluid, for practical purposes and in most situations, the differences are clinically irrelevant. No demonstrable difference in hemodynamic effect, morbidity, or mortality exists between resuscitation with either NS or RL. The amount of intravascular fluid requirements are related to the degree of vascular endothelial injury and impaired vasomotor tone; thus, not only may very large quantities of fluids be required initially, but continual fluid resuscitation often is required during the initial days of management of these patients.Dosing
Adult1-2 L IV initially, with reassessment of hemodynamic response; amounts required during the first few hours typically are 4-5 L
Pediatric
Not established
Interactions
None reported
Contraindications
Pulmonary edema in which added fluid promotes more edema and may lead to development of ARDS
Precautions
Pregnancy
A - Fetal risk not revealed in controlled studies in humans
Precautions
Closely monitor cardiovascular and pulmonary function; stop fluids when the desired hemodynamic response is observed or pulmonary edema develops; interstitial edema is a major complication; edema in an extremity is unsightly but not a significant complication; edema in brain or lungs is potentially fatal
Colloids
Resuscitation fluids used because they provide an oncotically active substance that expands plasma volume to a greater degree than isotonic crystalloids and reduces the tendency to pulmonary and cerebral edema. Approximately 50% of the administered colloid remains intravascular.
Albumin 5% (Albuminar, Albunex)
Used for treatment of certain types of shock or impending shock. Useful for plasma volume expansion and maintenance of cardiac output.
Solution of normal saline and 5% albumin is available for volume resuscitation.
Dosing
Adult
250-500 mL IV over 20-30 min, with reassessment of hemodynamic response
Pediatric
Not established
Interactions
None reported
Contraindications
Documented hypersensitivity; pulmonary edema; protein load of 5% albumin
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
No proven benefit of colloid resuscitation over isotonic crystalloids is known; protein load tends to exacerbate renal insufficiency, a potential complication of septic shock; studies document an increased incidence of renal failure in patients with colloid resuscitation
Antibiotics
Empirical antibiotics that cover the infecting organism and are started early are the only other proven medical treatment for septic shock. In order to provide the necessary coverage, broad-spectrum and/or multiple antibiotics are started. Monotherapy is possible in adults who are not immunocompromised with either antipseudomonal penicillin or a carbapenem. Combination therapy in adults involves 1 of the following: a third-generation cephalosporin plus anaerobic coverage (clindamycin or metronidazole) or a fluoroquinolone plus clindamycin. Administer all initial antibiotics intravenously in patients with septic shock.
Cefotaxime (Claforan)
Used for treatment of septicemia. Also used for treatment of gynecologic infections caused by susceptible organisms. Third-generation cephalosporin with enhanced gram-negative coverage, especially to Escherichia coli, Proteus species, and Klebsiella species. Has variable activity against Pseudomonas species.
Dosing
Adult
1-2 g IV q4h
Pediatric
Not established
Interactions
Probenecid may decrease cefotaxime clearance, causing an increase in cefotaxime levels; furosemide and aminoglycosides may increase nephrotoxicity when used concurrently
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Adjust dose in severe renal impairment; associated with severe colitis
Ceftriaxone (Rocephin)
Used because of an increasing prevalence of penicillinase-producing microorganisms. Inhibits bacterial cell wall synthesis by binding to 1 or more of the penicillin-binding proteins. Bacteria eventually lyse due to the ongoing activity of cell wall autolytic enzymes while cell wall assembly is arrested.
Dosing
Adult
1 g IV q6-12h
Pediatric
Not established
Interactions
Probenecid may decrease ceftriaxone clearance, causing an increase in ceftriaxone levels; ethacrynic acid, furosemide, and aminoglycosides may increase nephrotoxicity when used concurrently
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Adjust dose in renal impairment; use with caution in breastfeeding women and in patients allergic to penicillin
Cefuroxime (Zinacef)
Second-generation cephalosporin that maintains gram-positive activity of the first-generation cephalosporins and adds activity against E coli, Klebsiella pneumoniae, Proteus mirabilis, Haemophilus influenzae, and Moraxella catarrhalis. Condition of the patient, severity of the infection, and susceptibility of the microorganism determine proper dose and route of administration.
Dosing
Adult
1.5 g IV q8h
Pediatric
Not established
Interactions
Alcoholic beverages consumed concurrently within <72 h after taking cefuroxime may produce acute alcohol intolerance (disulfiramlike reaction); hypoprothrombinemic effects of anticoagulants may be increased by cephalosporins with the methyltetrazolethiol side chain (eg, cefuroxime); monitor renal function in patients receiving potent diuretics (eg, loop diuretics), risk of nephrotoxicity may be increased; aminoglycoside nephrotoxicity may potentiate cefuroxime effects in the kidney when used concurrently, monitor renal function closely
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Administer one half the dose to patients with creatinine clearance of 10-30 mL/min; administer one fourth the dose to patients with a creatinine clearance of <10 mL/min; use of antibiotics for prolonged periods of time or repeated therapy may result in bacterial or fungal overgrowth of nonsusceptible organisms that may lead to a secondary infection, take appropriate measures if superinfection occurs
Ticarcillin/clavulanate (Timentin)
Antipseudomonal penicillin plus a beta-lactamase inhibitor that provides coverage against most gram-positives (variable coverage against Staphylococcus epidermidis and none against methicillin-resistant Staphylococcus aureus [MRSA]), most gram-negative organisms, and most anaerobes.
Dosing
Adult
3.1 g (ticarcillin 3 g and claculanate 0.1 g) IV q4-6h
Pediatric
Not established
Interactions
Tetracyclines may decrease effects; high concentrations may physically inactivate aminoglycosides if administered in the same IV line; probenecid may increase penicillin levels; effects when administered concurrently with aminoglycosides are synergistic
Contraindications
Documented hypersensitivity; severe pneumonia; do not treat bacteremia, pericarditis, emphysema, meningitis, and purulent or septic arthritis with an oral penicillin during acute stage
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Perform CBCs prior to initiation of therapy and at least weekly during therapy; monitor for liver function abnormalities by measuring AST and ALT during therapy; perform urinalysis, BUN, and creatinine determinations during therapy, and adjust dose if these values become elevated; if renal impairment is known or suspected, adjust dose and monitor blood levels; these measures avoid possible neurotoxic reactions
Piperacillin/tazobactam (Zosyn)
Inhibits the biosynthesis of cell wall mucopeptide and is effective during the stage of active multiplication. Has antipseudomonal activity.
Dosing
Adult
3/0.375 g (piperacillin 3 g and tazobactam 0.375 g) IV q6h
Pediatric
Not established
Interactions
Tetracyclines may decrease the effects; high concentrations may physically inactivate aminoglycosides; probenecid may increase penicillin levels; effects when administered concurrently with aminoglycosides are synergistic
Contraindications
Documented hypersensitivity; do not treat severe pneumonia, bacteremia, pericarditis, emphysema, meningitis, and purulent or septic arthritis with an oral penicillin during the acute stage
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Perform CBCs prior to initiation of therapy and at least weekly during therapy; monitor for liver function abnormalities by measuring AST and ALT during therapy; perform urinalysis, BUN, and creatinine determinations during therapy, and adjust dose if these values become elevated; if renal impairment is known or suspected, adjust dose and monitor blood levels; these measures avoid possible neurotoxic reactions
Imipenem and cilastatin (Primaxin)
Carbapenem with activity against most gram-positive organisms (except MRSA), gram-negative organisms, and anaerobes. Used for treatment of multiple organism infections in which other agents do not have wide spectrum coverage or are contraindicated due to their potential for toxicity.
Dosing
Adult
500 mg IV q6h
Pediatric
<12 years: Not established
>12 years: Administer as in adults
Interactions
Coadministration with cyclosporine may increase CNS adverse effects of both agents; coadministration with ganciclovir may result in generalized seizures
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Adjust dose in renal insufficiency; avoid use in children <12 years
Meropenem (Merrem)
Carbapenem with slightly increased activity against gram-negative organisms and slightly decreased activity against staphylococci and streptococci compared to imipenem.
Dosing
Adult
1 g IV q8h
Pediatric
Not established
Interactions
Probenecid may inhibit renal excretion of meropenem, increasing meropenem levels
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Pseudomembranous colitis and thrombocytopenia may occur, requiring immediate discontinuation of medication
Clindamycin (Cleocin)
Primarily used for its activity against anaerobes. Has some activity against streptococcus and methicillin-sensitive S aureus (MSSA).
Dosing
Adult
600-900 mg IV q8h
Pediatric
5-10 mg/kg IV q8h
Interactions
Increases duration of neuromuscular blockade induced by tubocurarine and pancuronium
Contraindications
Documented hypersensitivity; regional enteritis; ulcerative colitis; hepatic impairment; antibiotic-associated colitis
Precautions
Pregnancy
D - Fetal risk shown in humans; use only if benefits outweigh risk to fetus
Precautions
Dose adjustment may be necessary in severe hepatic dysfunction; no adjustment is necessary in renal insufficiency; associated with severe and possibly fatal colitis
Metronidazole (Flagyl)
Imidazole ring-based antibiotic active against various anaerobic bacteria and protozoa. Usually employed in combination with other antimicrobial agents, except when it is used for Clostridium difficile enterocolitis in which monotherapy is appropriate.
Dosing
Adult
Loading dose: Infuse 15 mg/kg IV over 1 h or 1 g for a 70-kg adult
Maintenance dose: Infuse 7.5 mg/kg IV over 1 h q6-8h or 500 mg for a 70-kg adult; administer 6 h following the loading dose; not to exceed 4 g in 24 h
Pediatric
Not established
Interactions
Potentiates anticoagulant effect of warfarin; agents that alter hepatic P450 system affect its clearance; phenytoin and phenobarbital may decrease half-life; may reduce metronidazole clearance and increase toxicity; may increase effect of anticoagulants; may decrease lithium and phenytoin clearance, increasing their toxicity; disulfiramlike reactions may occur when used concurrently with orally ingested ethanol (although the risk for most patients may be slight, exercise caution)
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Adjust dose in patients with severe hepatic disease because they may metabolize metronidazole slowly; monitor patients for seizures and the development of peripheral neuropathy
Ciprofloxacin (Cipro)
Fluoroquinolone that inhibits bacterial DNA synthesis and, consequently, growth, by inhibiting DNA gyrase and topoisomerases, which are required for replication, transcription, and translation of genetic material. Quinolones have broad activity against gram-positive and gram-negative aerobic organisms. Has no activity against anaerobes. Continue treatment for at least 2 d (7-14 d typical) after signs and symptoms have disappeared.
Dosing
Adult
400 mg IV q12h
Pediatric
10-15 mg/kg IV q12h
Interactions
Antacids, iron salts, and zinc salts may reduce serum levels; administer antacids 2-4 h before or after taking fluoroquinolones; cimetidine may interfere with metabolism of fluoroquinolones; ciprofloxacin reduces therapeutic effects of phenytoin; probenecid may increase ciprofloxacin serum concentrations
May increase toxicity of theophylline, caffeine, cyclosporine, and digoxin (monitor digoxin levels); may increase effects of anticoagulants (monitor PT)
Contraindications
Documented hypersensitivity
Precautions
Pregnancy
C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Dosage adjustments (adult adjustments)
CrCl (mL/min) <10: 50% of PO or IV dose q12h
HD: 0.25-0.5 g PO or 0.2-0.4 g IV q12h
During peritoneal dialysis: 0.25-0.5 g PO or 0.2-0.4 g IV q8h
In prolonged therapy, perform periodic evaluations of organ system functions (eg, renal, hepatic, hematopoietic); adjust dose in renal function impairment; superinfections may occur with prolonged or repeated antibiotic therapy
Not drug of first choice in pediatrics due to increased incidence of adverse events compared to controls, including arthropathy; no data exist for dose for pediatric patients with renal impairment (ie, CrCl <50 mL/min)
Fluoroquinolones are associated with increased risk of tendinitis and tendon rupture in all ages, this risk is further increased in older patients usually over 60 y, in patients taking corticosteroid drugs, and in patients with kidney, heart, or lung transplants
Activated protein C analogs
Exert antithrombic effects, have indirect profibrinolytic activity, and may have anti-inflammatory effect.
Drotrecogin alfa (Xigris)
Indicated for reduction of mortality in patients with severe sepsis associated with acute organ dysfunction and at high risk of death. Recombinant form of human activated protein C that exerts antithrombotic effect by inhibiting factors Va and VIIIa. Has indirect profibrinolytic activity by inhibiting plasminogen activator inhibitor-1 (PAI-1) and limiting formation of activated thrombin-activatable-fibrinolysis-inhibitor. May exert anti-inflammatory effect by inhibiting human tumor necrosis factor (TNF) production by monocytes, blocking leukocyte adhesion to selectins, and limiting thrombin-induced inflammatory responses within microvascular endothelium.
Dosing
Adult
24 mcg/kg/h IV continuous infusion for 96 h; ideally, initiate within 48 h of sepsis onset
Pediatric
Not established
Interactions
None reported; coadministration with drugs that affect hemostasis may increase risk of bleeding (eg, warfarin, heparin, thrombolytics, glycoprotein IIb/IIIa inhibitors)
Contraindications
Documented hypersensitivity; increased risk of bleeding (eg, active internal bleeding, recent hemorrhagic stroke, recent intraspinal or intracranial surgery, recent or current trauma, presence of epidural catheter, intracranial neoplasm, cerebral herniation, severe head trauma)
Precautions
Pregnancy
C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Bleeding is most common serious adverse effect; caution with conditions that increase risk of bleeding including INR >3, concurrent therapeutic heparin (>15 U/kg/h), within 6 wk of GI bleeding episode, within 3 d of thrombolytic therapy, within 7 d of platelet inhibitors administration, within 3 mo of ischemic stroke, intracranial arteriovenous malformation or aneurysm, known bleeding diathesis, chronic severe hepatic disease; stop infusion if clinically significant bleeding occurs
Vasopressor supportive therapy
If patient does not respond to several liters of isotonic crystalloid (usually 4 or more) or evidence of volume overload is present, the depressed cardiovascular system can be stimulated by inotropic and vasoconstrictive agents.
Dopamine (Inotropin)
Used to treat hypotension in fluid-resuscitated patients. Stimulates both adrenergic and dopaminergic receptors. Hemodynamic effect depends on the dose. Lower doses stimulate mainly dopaminergic receptors that produce renal and mesenteric vasodilation in health volunteers, but probably have no measurable effect in patients who are critically ill. Higher doses produce cardiac stimulation, tachycardia, and vasoconstriction.
Dosing
Adult
In hypotensive hyperdynamic shock: starting dose of 2-5 mcg/kg/min IV; titrate as needed to maintain a MAP >60 mm Hg; may be increased by 1-4 mcg/kg/min q10-30min IV until satisfactory response; not to exceed 20 mcg/kg/min
Maintenance dose: <20 mcg/kg/min IV usually are adequate for 50% of patients treated
Pediatric
Not established
Interactions
Phenytoin, alpha-adrenergic and beta-adrenergic blockers, general anesthesia, and MAO inhibitors increase and prolong the effects
Contraindications
Documented hypersensitivity; tachycardia; pheochromocytoma; ventricular tachyarrhythmias
Precautions
Pregnancy
C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Monitor urine flow, cardiac output, pulmonary wedge pressure, and BP closely during the infusion; prior to infusion, correct hypovolemia with either whole blood or plasma, as indicated; monitoring of central venous pressure or left ventricular filling pressure may be helpful in detecting and treating hypovolemia
Norepinephrine (Levophed)
As with dopamine, it is used to treat hypotension following adequate fluid-volume replacement. Norepinephrine stimulates beta 1-adrenergic and alpha-adrenergic receptors, which increase arterial tone and cardiac contractility. As a result, systemic BP and coronary blood flow increases with norepinephrine, though myocardial oxygen demand also may increase. After obtaining a response, adjust infusion rate to maintain a MAP greater than 60 mm Hg. BP levels below this threshold are insufficient to perfuse vital organs while increasing pressures much greater than 70 mm Hg, using vasopressors does not further increase tissue blood flow.
Dosing
Adult
0.05-2 mcg/kg/min IV; titrate to effect
Pediatric
Not established
Interactions
Atropine sulfate may enhance pressor response of norepinephrine by blocking reflex bradycardia caused by norepinephrine
Contraindications
Documented hypersensitivity; known hypovolemia; peripheral or mesenteric vascular thrombosis because ischemia may be increased and the area of the infarct extended
Precautions
Pregnancy
D - Fetal risk shown in humans; use only if benefits outweigh risk to fetus
Precautions
Correct blood-volume depletion, if possible, before administering norepinephrine therapy; extravasation may cause severe tissue necrosis, administer into a large vein; use with caution in patients with occlusive vascular disease
Vasopressin (Pitressin)
Has vasopressor and antidiuretic hormone (ADH) activity. Although vasopressin does not increase BP in healthy subjects, it markedly increases vasomotor tone in patients with septic shock. It also increases water resorption at the distal renal tubular epithelium (ADH effect) and promotes smooth muscle contraction throughout the vascular bed of the renal tubular epithelium (vasopressor effects). Vasoconstriction also is increased in splanchnic, portal, coronary, cerebral, peripheral, pulmonary, and intrahepatic vessels. Vasopressin is not yet routinely used to treat hypotension in septic shock. The dosage of vasopressin used for hypotension is one-tenth that used to treat upper GI bleeding from varices.
Dosing
Adult
Suggested dose: 0.01-0.05 U/min IV; titrate dose as needed
Pediatric
Not established
Interactions
Lithium, epinephrine, demeclocycline, heparin, and alcohol may decrease the effects of vasopressin; conversely, chlorpropamide, urea, fludrocortisone, and carbamazepine potentiate its effects
Contraindications
Documented hypersensitivity; coronary artery disease
Precautions
Pregnancy
B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Caution in cardiovascular disease, seizure disorders, nitrogen retention, asthma, or migraine; excessive doses may result in hyponatremia
Follow-up
Further Inpatient Care
- The major focus of resuscitation from septic shock is supporting cardiac and respiratory functions. To prevent multi-organ failure, these patients require a very close monitoring and institution of appropriate therapy for major organ function. Some of the problems encountered in these patients are the following:
- Temperature control
- Fever generally requires no treatment, except in patients with limited cardiovascular reserve, because of increased metabolic requirements.
- Antipyretic drugs and physical cooling methods, such as sponging or cooling blankets, may be used to lower the temperature.
- Metabolic support
- Patients with septic shock develop hyperglycemia and electrolyte abnormalities.
- Serum glucose should be kept in normal range with insulin infusion. Regular measurement and correction of electrolyte deficiency including hypokalemia, hypomagnesemia, hypocalcemia and hypophosphatemia is recommended.
- Anemia and coagulopathy
- Hemoglobin as low as 80 mg/dL is well tolerated and does not require transfusion unless the patient has poor cardiac reserve or demonstrates evidence of myocardial ischemia.
- Thrombocytopenia and coagulopathy are common in sepsis and do not require replacement with platelets or fresh frozen plasma, unless the patient develops active clinical bleeding.
- Renal dysfunction
- Closely monitor urine output and renal function in all patients who are septic.
- Any abnormalities of renal function should prompt attention to adequacy of circulating blood volume, cardiac output, and BP; correct these if they are inadequate.
- Nutritional support
- Early nutritional support is of critical importance in patients with septic shock. The enteral route is preferred unless the patient has an ileus or other abnormality.
- Gastroparesis is observed commonly and can be treated with motility agents or placement of a small bowel feeding tube.
Transfer
If patients are treated initially in the wards or in the emergency department, after initial attempts at stabilization, transfer them to the ICU for invasive monitoring and support.
Deterrence/Prevention
- Patients with impaired host defense mechanisms are at a greatly increased risk for developing sepsis and multiorgan failure. The main causes are: chemotherapeutic drugs, malignancy, severe trauma, burns, diabetes mellitus, renal or hepatic failure, old age, ventilatory support, and invasive catheters.
- The development of severe sepsis may be prevented by avoidance of invasive catheters or removing them as soon as possible. Prophylactic antibiotics in the perioperative phase, particularly following GI surgery, may be beneficial. Use of topical antibiotics around invasive catheters and as part of a dressing for patients with burns is helpful. Maintenance of adequate nutrition, pneumococcal vaccine in patients who have had a splenectomy, and early enteral feeding are other preventive measures.
- Prevention of sepsis and multiorgan failure with topical or systemic antibiotics in patients who are at high risk: The use of nonabsorbable antibiotics in the stomach to prevent translocation of bacteria and occurrence of bacteremia has been a controversial issue. Numerous trials have been performed over the years using either the topical antibiotics alone or a combination of topical and systemic antibiotics. A systemic review by Nathens presented no benefit in medical patients but a reduced mortality in surgical trauma patients. The beneficial effect was from a combination of systemic and topical antibiotics, predominantly by reducing lower respiratory tract infections in patients who were treated.
Prognosis
- Several clinical trials have demonstrated a mortality ranging from 40-75% in patients with multiorgan failure of sepsis.
- The poor prognostic factors are advanced age, infection with a resistant organism, impaired host immune status, and poor prior functional status.
- Development of sequential organ failure, despite adequate supportive measures and antimicrobial therapy is a harbinger of a poor outcome.
- In one study, mortality rates were 7% with SIRS, 16% with sepsis, 20% with severe sepsis, and 46% with septic shock.
- A multicenter prospective study published in JAMA reported a mortality of 56% during ICU stay. Of all deaths, 27% occurred within 2 days of the onset of severe sepsis, and 77% of all deaths occurred within the first 14 days. The risk factors for early mortality in this study were a higher severity of illness score, presence of 2 or more acute organ failures at the time of sepsis, shock, and a low blood pH (<7.3).
Patient Education
For excellent patient education resources, visit eMedicine's Blood and Lymphatic System Center. Also, see eMedicine's patient education article Sepsis (Blood Infection).
Miscellaneous
Medicolegal Pitfalls
- Sepsis is the most common cause of shock leading to multiorgan failure in most ICUs and is a leading cause of death.
- Recognition of septic shock requires features of SIRS (mental changes; hyperventilation; distributive hemodynamics; hyperthermia; hypothermia; and reduced, elevated, or left-shift on WBC) and existence of a potential source of infection.
- Patients in septic shock require immediate cardiorespiratory stabilization with large volume of fluids intravenously, infusion of vasoactive drugs, and often, endotracheal intubation and mechanical ventilation.
- Immediately direct intravenous empirical antibiotic therapy at all potential infectious sources.
- Infectious processes require drainage or debridement surgically and expeditiously, even if the patient does not appear stable because the patient may not improve without emergent surgical treatment.
- The effects of drugs used to support the hemodynamics of patients who are septic have adverse effects on splanchnic circulation. Therefore, the ideal hemodynamic therapy in these patients is not known. Following adequate fluid resuscitation, therapy with dopamine may be initiated, followed by norepinephrine when dopamine fails. The alternate approach is initiating therapy with norepinephrine and using dobutamine if inotropic support is needed.
- Manipulation of oxygen delivery by increasing cardiac index has either shown no improvement or has worsened morbidity and mortality. Routine use of hemodynamic drugs to improve cardiac output to supranormal is not recommended.
- Epinephrine use in septic shock as a single agent is not recommended. Epinephrine impairs splanchnic circulation and tissue perfusion.
- Lactic acidosis of septic shock usually causes anion gas metabolic acidosis. Administration of bicarbonate therapy has a potential for worsening of intracellular acidosis. Correction of acidemia using sodium bicarbonate has not been proven to improve hemodynamics in patients who are critically ill with increased blood lactate. The above not withstanding, bicarbonate therapy has been used for pH less than 7.20 or bicarbonate less than 9 mmol/L, though no data to support this practice exist.
Special Concerns
- A continuum of severity from sepsis to septic shock and multiorgan failure exists. The clinical spectrum usually begins with infection that potentially leads to sepsis and organ dysfunction. In one study with 2527 patients evaluated, 26% developed sepsis, 18% developed severe sepsis, and 4% developed septic shock. The incidence of positive blood cultures was 17% in patients with sepsis and 69% in patients with septic shock.
- The pathogenesis of septic shock and multiorgan failure occurs from mediators produced because of the immune response of the host. Despite encouraging data from animal studies, immunosuppressive agents, such as high-dose corticosteroids, have not shown any benefit in humans.
- Recent research has focused on modifying the host response to sepsis by infusion of antibodies against gram-negative endotoxin, gamma globulins, monoclonal antibodies against tumor necrosis factor, blockade of eicosanoid production, blockade IL-1 activity, and inhibition of nitric oxide synthase. These approaches have demonstrated modest success in animal experimentation but cannot be recommended for general use at this time.
No comments:
Post a Comment